Breast cancer remains one of the most significant health challenges for women worldwide. In 2022, it caused around 670,000 deaths globally and remained the most common cancer in women in 157 out of 185 countries. These statistics underscore its profound impact, a reality that touches women from all walks of life. What’s particularly important to understand is that approximately 50% of cases occur in women without any specific risk factors other than their sex and age.
Understanding Your Risk
Knowledge is your first line of defense in managing breast cancer risk. Although the disease can affect anyone regardless of geographic location, background, or lifestyle, understanding risk factors empowers you to take proactive steps.
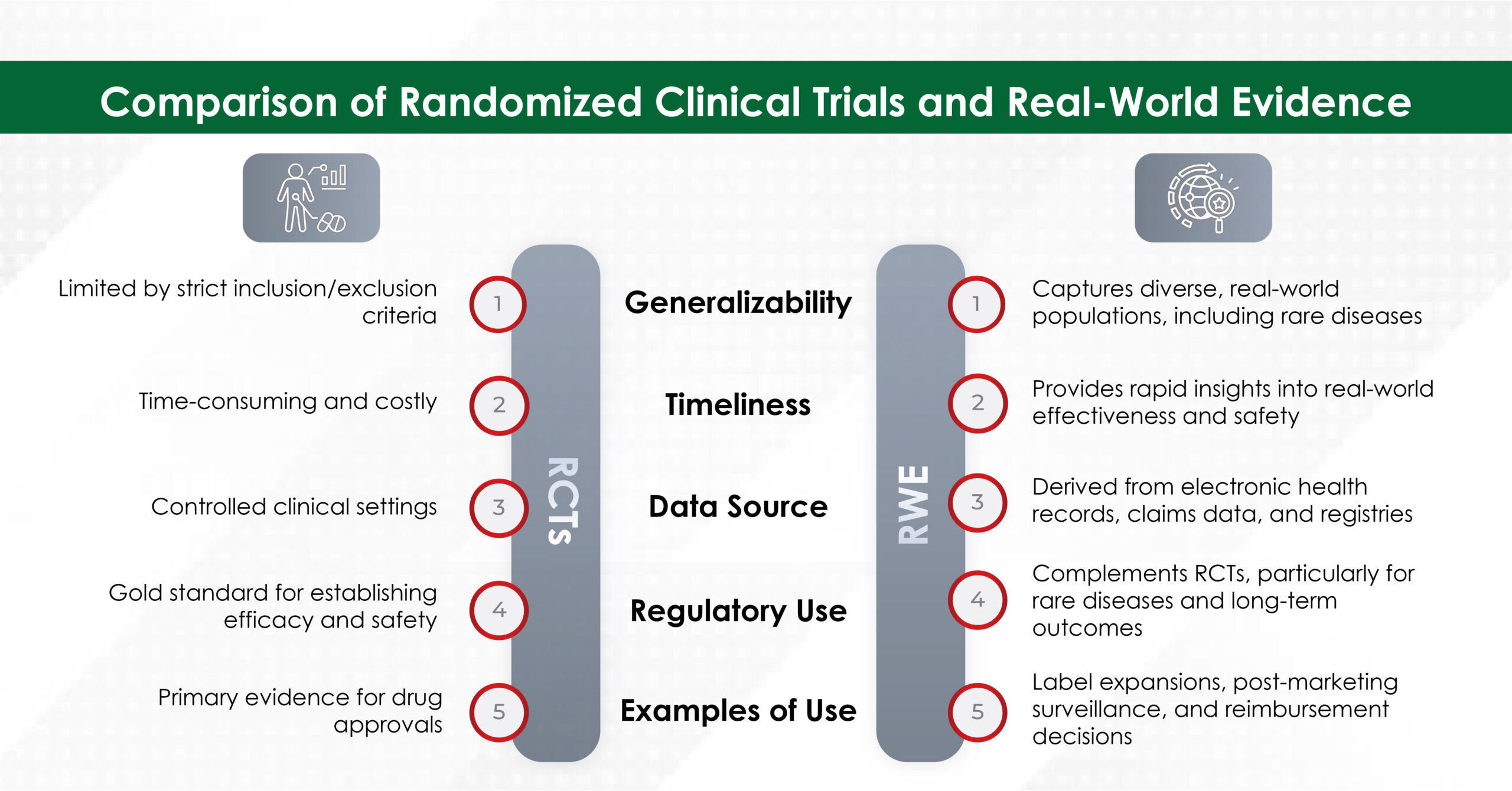
Key Risk Factors:
Being female is the strongest breast cancer risk factor. Beyond this, several factors can increase risk, including advancing age, obesity, harmful alcohol use, tobacco use, and family history of breast cancer. Your reproductive history matters too—factors such as the age when menstrual periods began, age at first pregnancy, and postmenopausal hormone therapy can all play a role. Additionally, a history of radiation exposure increases risk.
Family history deserves special attention. Certain inherited high-penetrance gene mutations greatly increase breast cancer risk, with mutations in the BRCA1, BRCA2, and PALB-2 being the most dominant.
Recognizing Symptoms: When to See Your Doctor
Breast cancer in its early stages often presents no symptoms, which is precisely why regular screening is vital. However, if you notice any of the following changes, consult with a healthcare provider promptly.
Changes warranting prompt healthcare provider attention
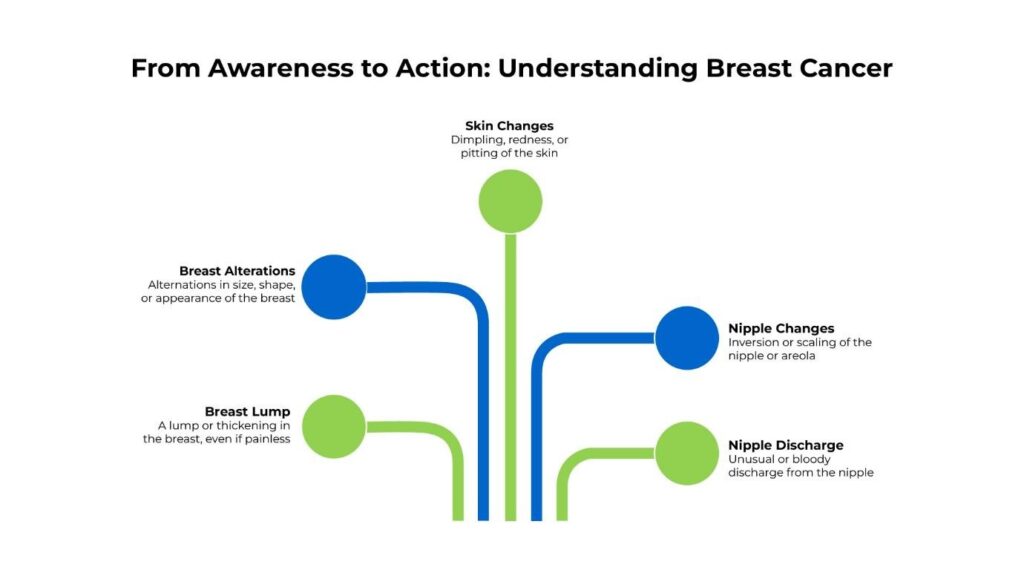
It’s reassuring to know that most breast lumps are not cancerous. However, any abnormal lump should be evaluated by a healthcare professional. Early detection leads to the best outcomes, as cancer is most treatable when it is small and localized.
Screening Guidelines: When and How Often
Understanding when to start screening and how often to undergo mammograms is crucial for early detection. The American Cancer Society provides the following recommendations for women at average risk:
- Ages 40–44 years: Mammograms may begin annually, depending on individual choice
- Ages 45–54 years: Annual mammograms are recommended
- Ages 55 years and older: Mammograms may be conducted every two years or continued annually, based on health and personal preference
Women at high risk of breast cancer should get both a breast MRI and a mammogram every year. High-risk categories include women with a personal or family history (especially first-degree relatives like parents, siblings, or children) of breast cancer, those with known BRCA gene mutations, or those with a history of chest radiation before age 30.
The Critical Importance of Early Detection
Early detection plays a transformative role in improving breast cancer outcomes. When combined with timely treatment and effective screening, it significantly reduces the global burden of this disease.
When breast cancer spreads, it can move to lymph nodes and other organs, including the lungs, liver, brain, and bones. Catching the cancer early, when it is localized, dramatically increases the chances of successful treatment—a fact that underscores why awareness and screening are so vital.
Don’t Hesitate: The Importance of Speaking Up
Many women experience hesitancy when it comes to discussing breast health concerns. You might feel embarrassed about a change you’ve noticed, unsure if it’s “serious enough” to warrant a doctor’s visit, or simply not know where to turn for help.
Remember: No concern is too small to discuss with your healthcare provider. Early conversations about changes in your breasts can lead to early detection. If you’re unsure where to start, your primary care physician can guide you or refer you to a specialist.
Clear communication between patients and healthcare providers is vital in the fight against breast cancer. This is where effective medical communication plays a crucial role, translating complex research into accessible information and ensuring that accurate health messages reach every woman who needs them. Don’t let hesitation prevent you from getting the care you need.
Summary
Breast cancer remains a significant global health challenge, but knowledge and early detection save lives. Understanding your risk factors, recognizing warning signs, and following appropriate screening guidelines are essential steps every woman can take. Whether you’re at average or high risk, regular mammograms and awareness of breast changes can make a crucial difference in outcomes. Remember, approximately 50% of breast cancer cases occur in women with no specific risk factors beyond sex and age—making awareness and screening vital for everyone. Open communication with your healthcare provider about any concerns is equally important. Early detection, combined with timely treatment, offers the best chance for successful outcomes.
At Turacoz, we specialize in transforming complex medical insights into clear, actionable information. By crafting compelling narratives that educate, engage, and empower, we aim to make a positive impact on patient outcomes. Contact us today to learn how we can support your healthcare messaging efforts.
References
- Breast cancer. Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer. Last accessed: October 2025.
- American Cancer Society Recommendations for the Early Detection of Breast Cancer. Available at: https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html. Last accessed: October 2025.
- Breast cancer risk factors. Available at: https://www.cdc.gov/breast-cancer/risk-factors/index.html. Last accessed: October 2025.























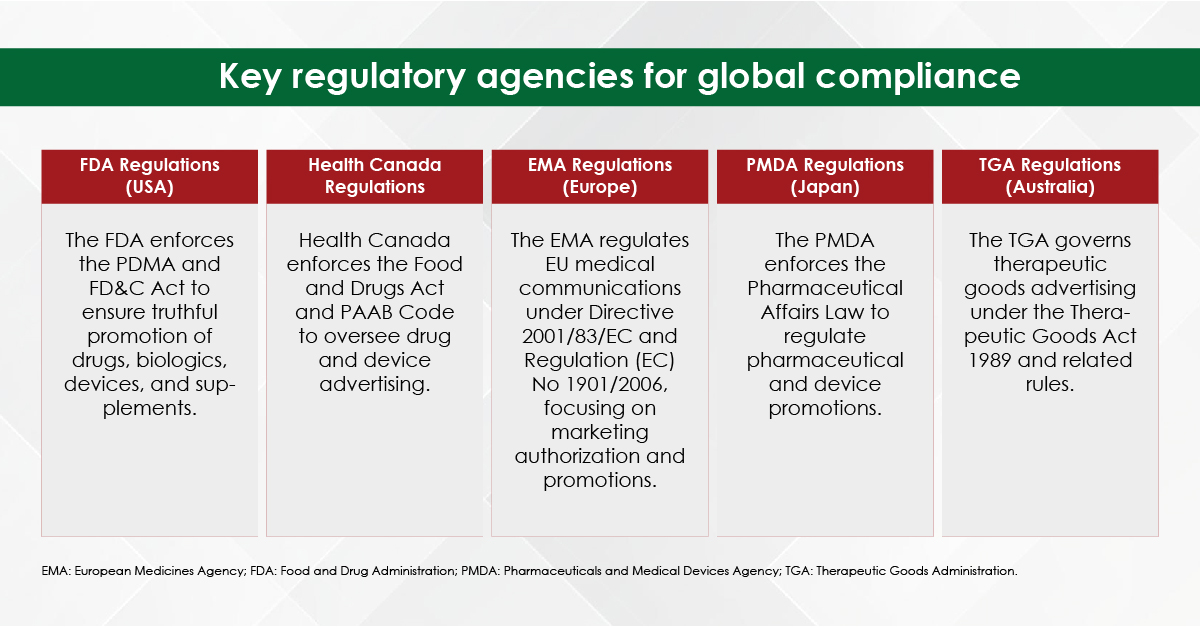
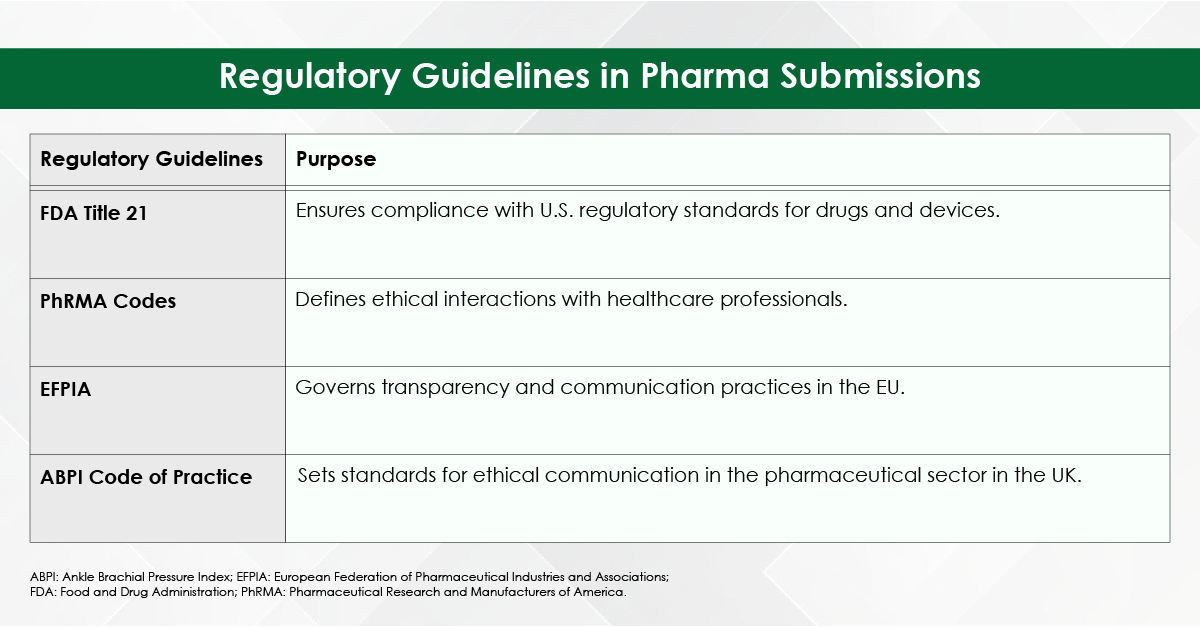

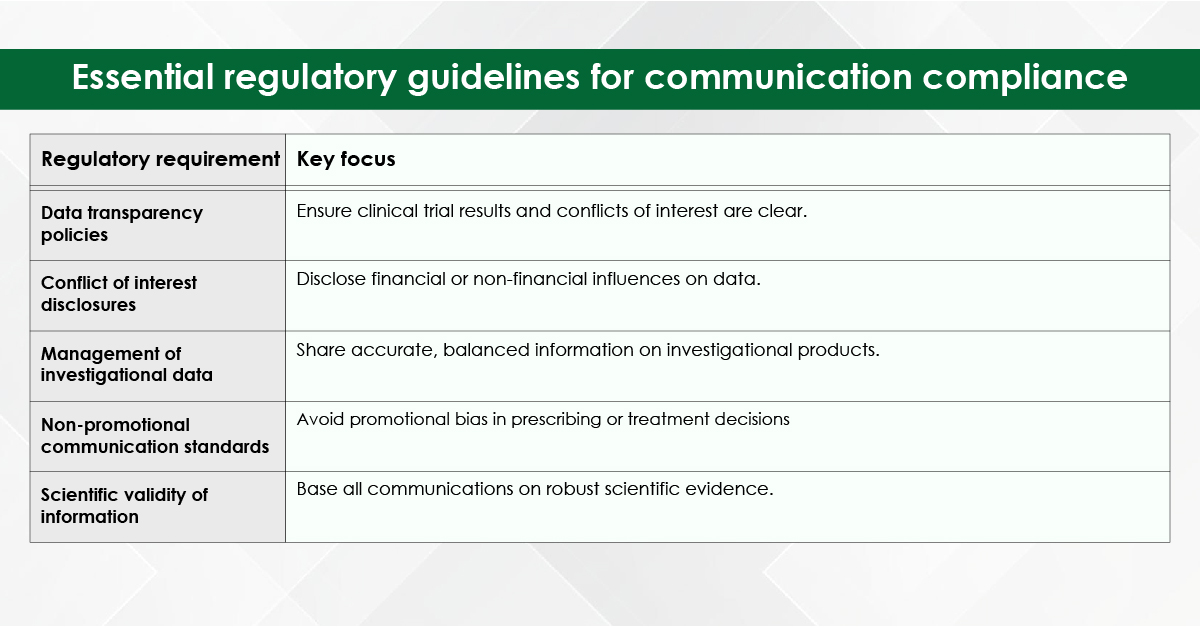
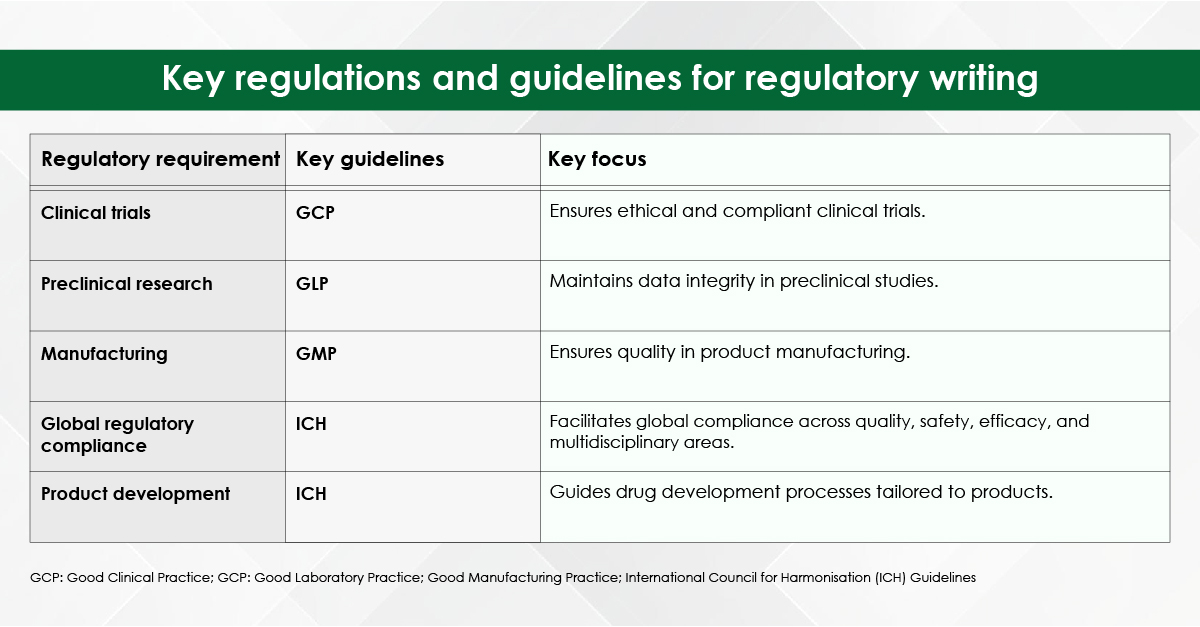

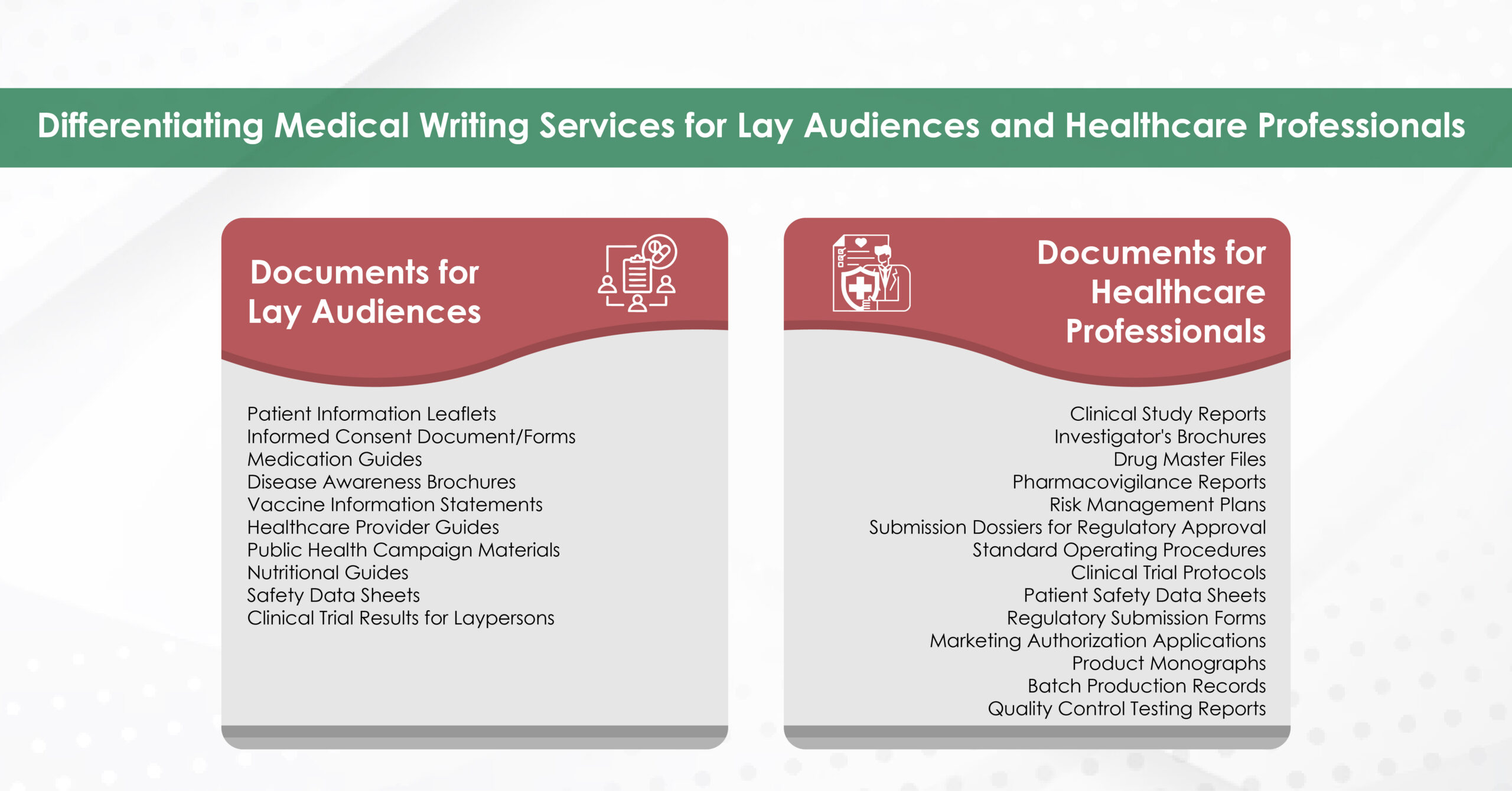




























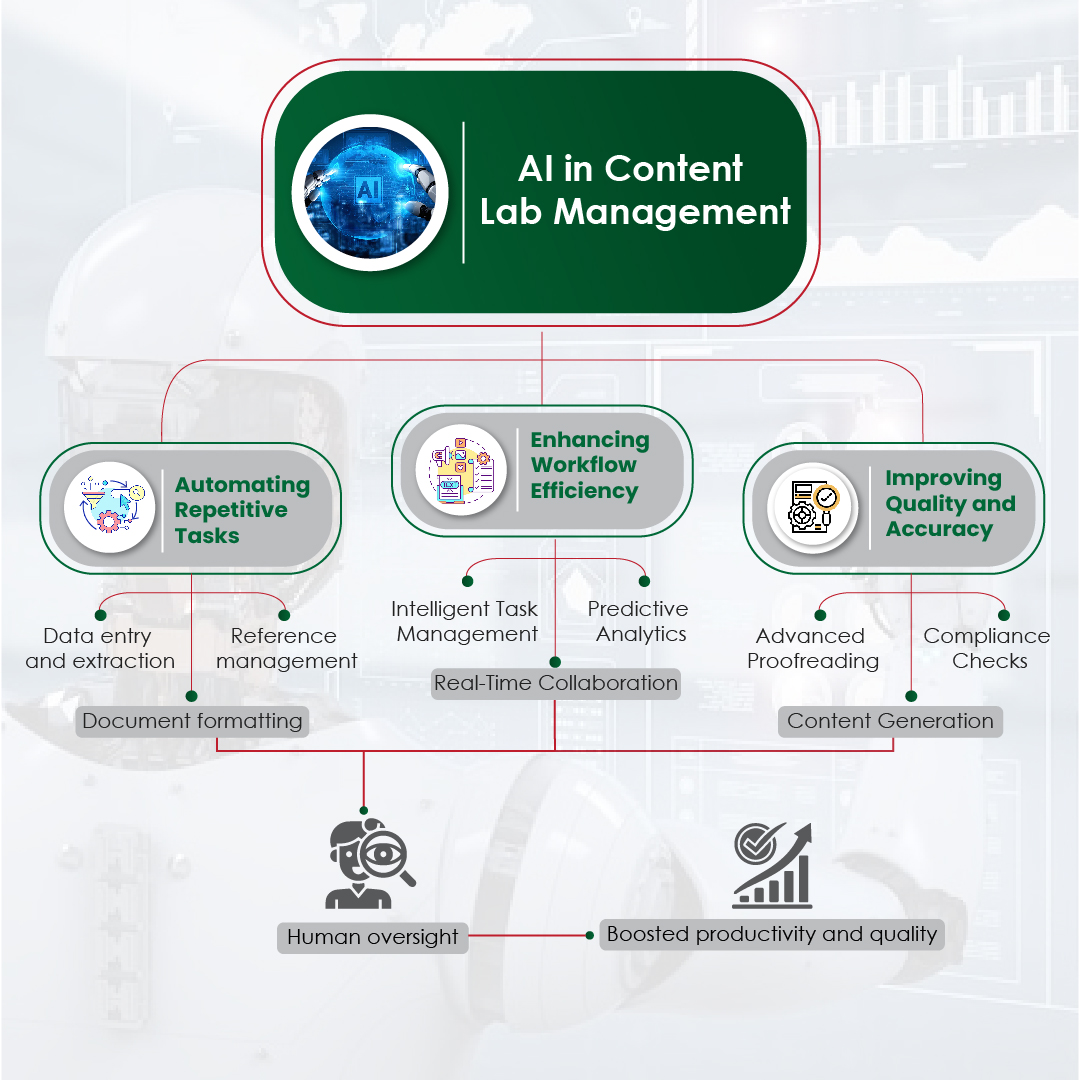
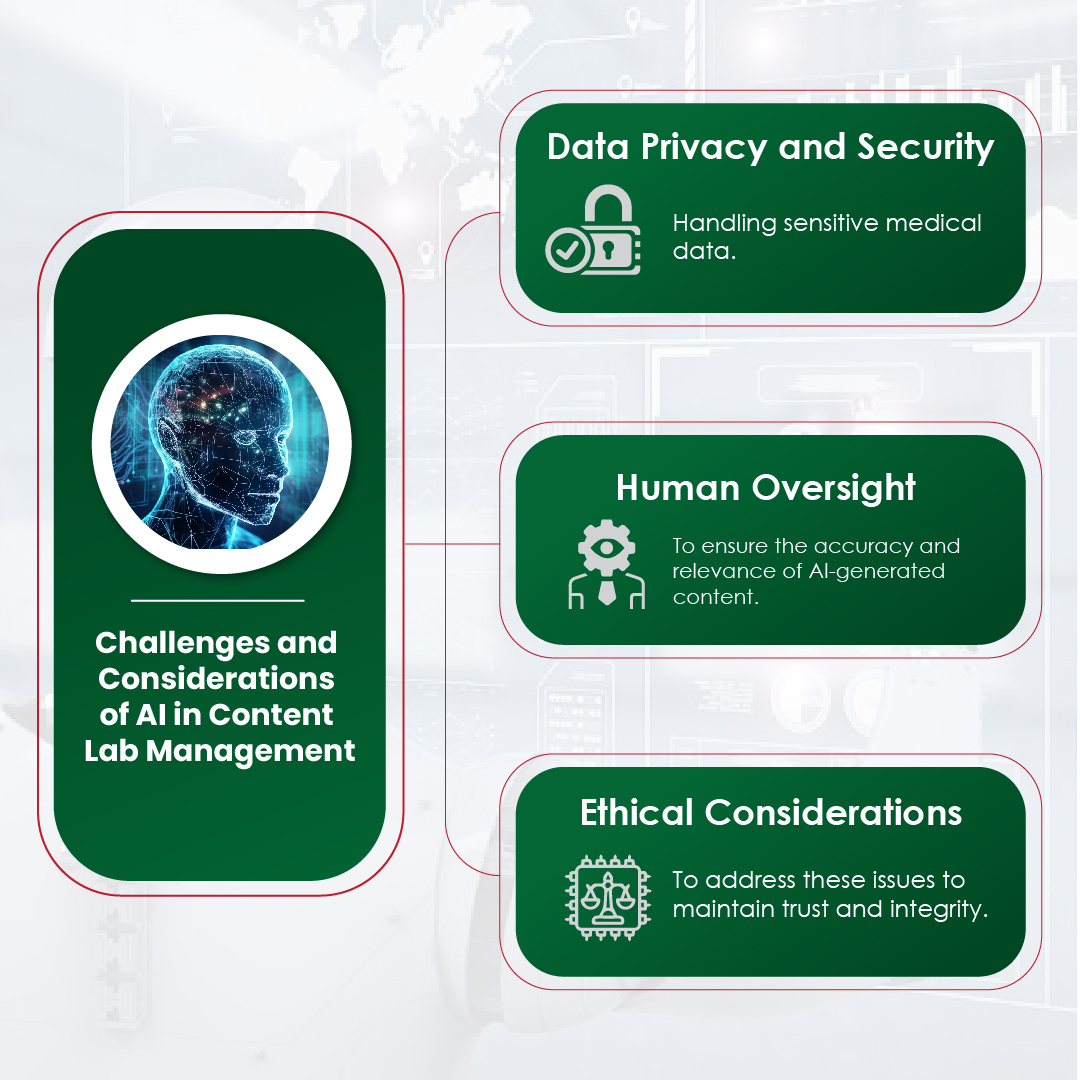

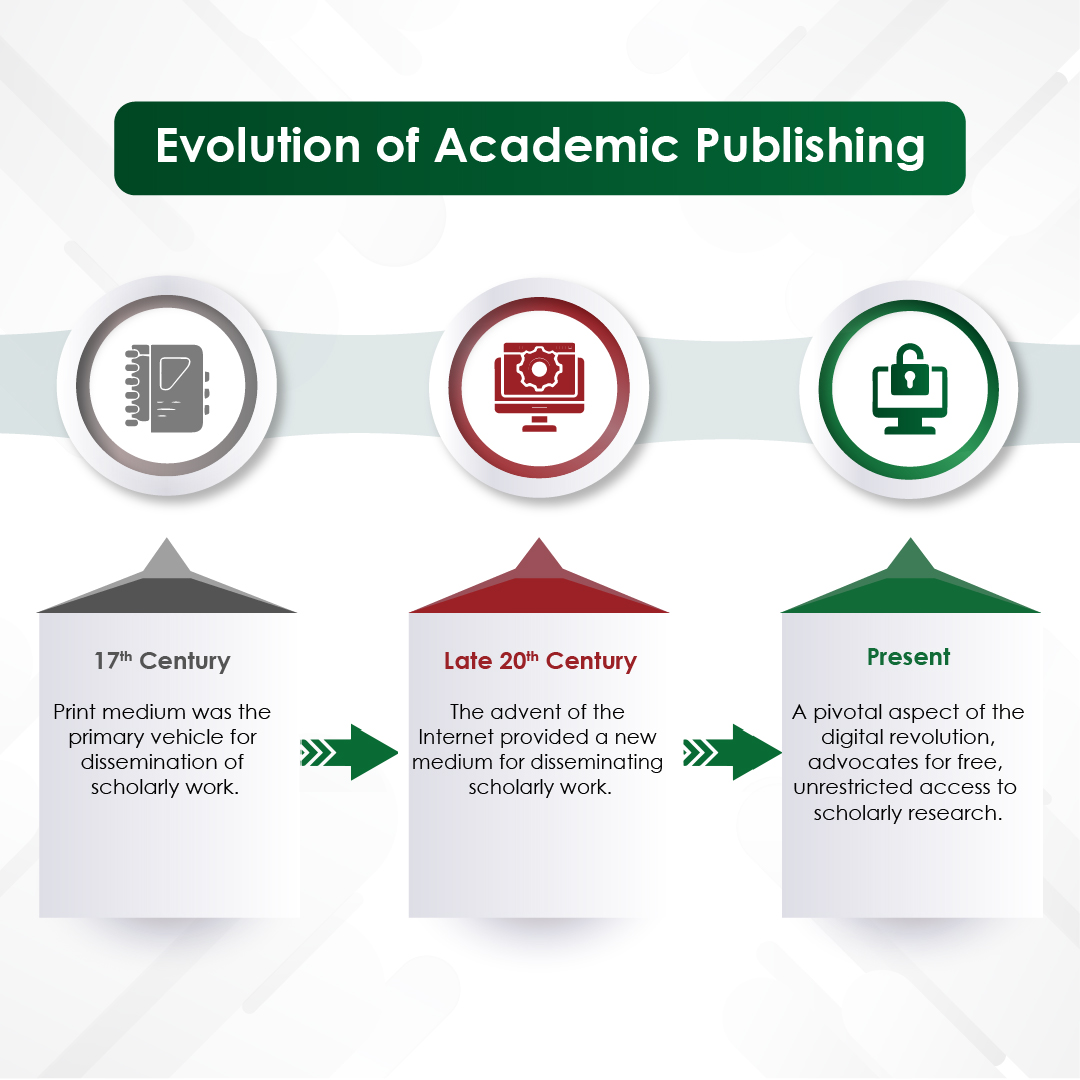

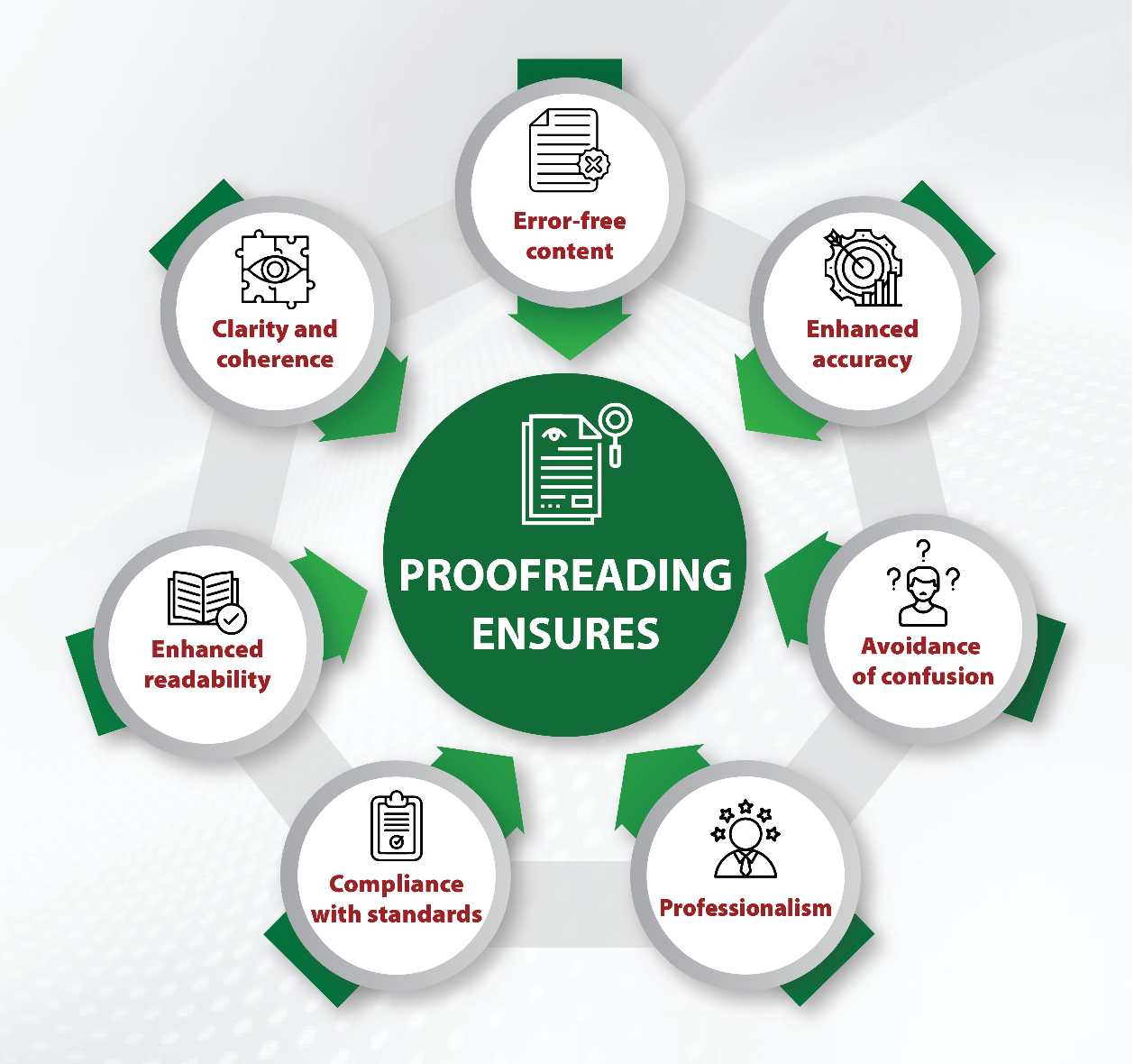


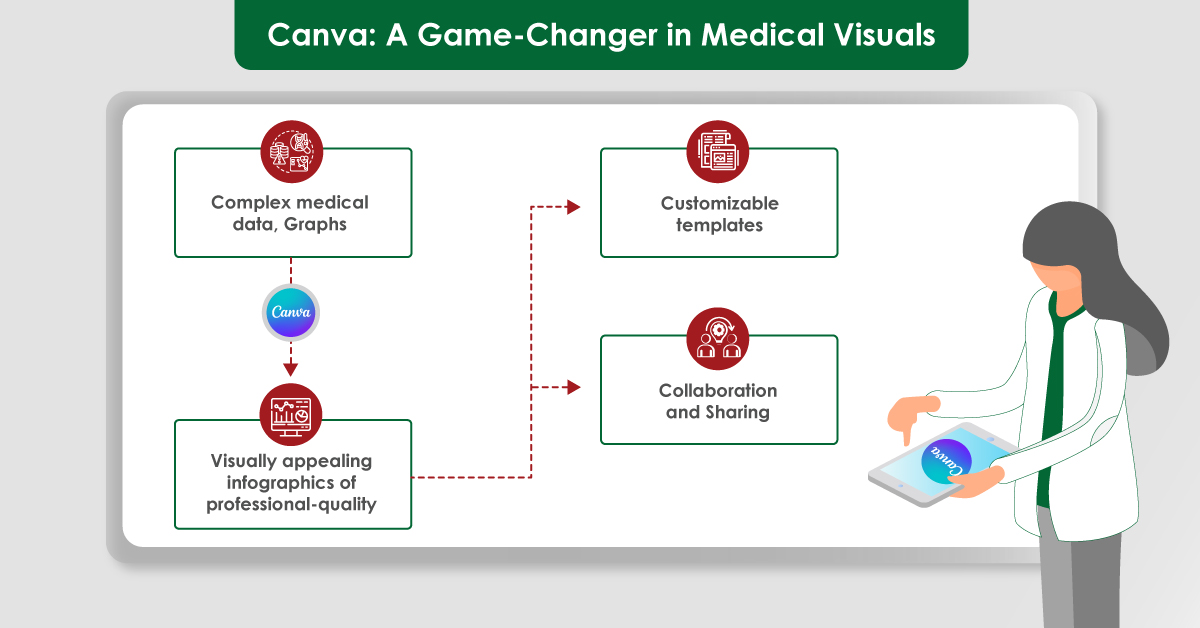
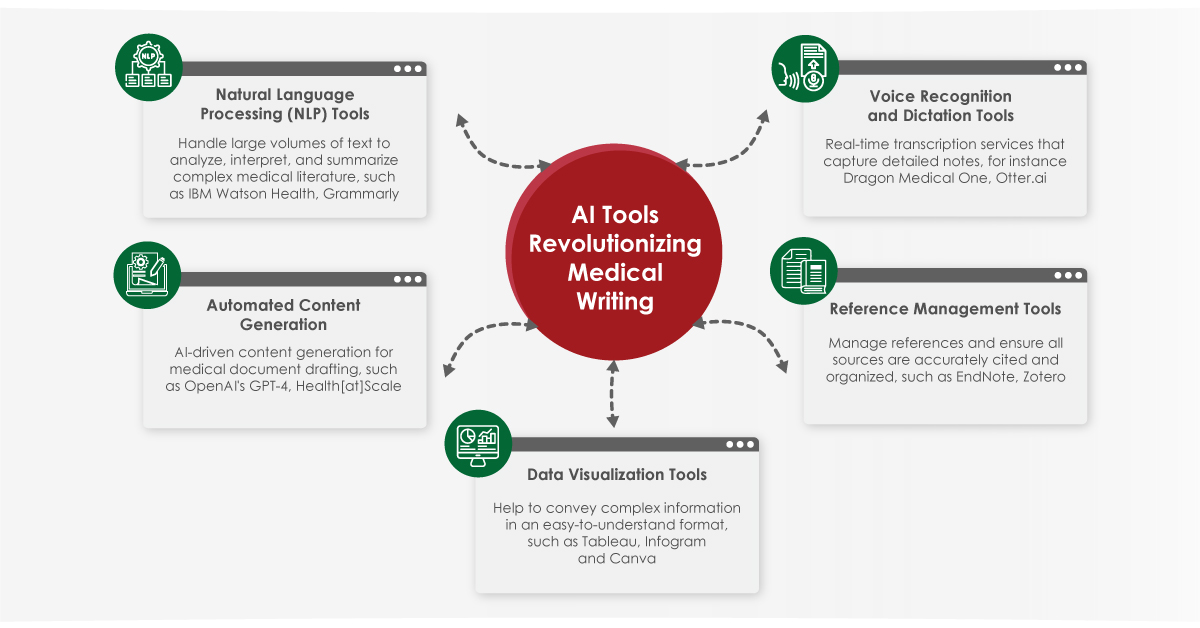



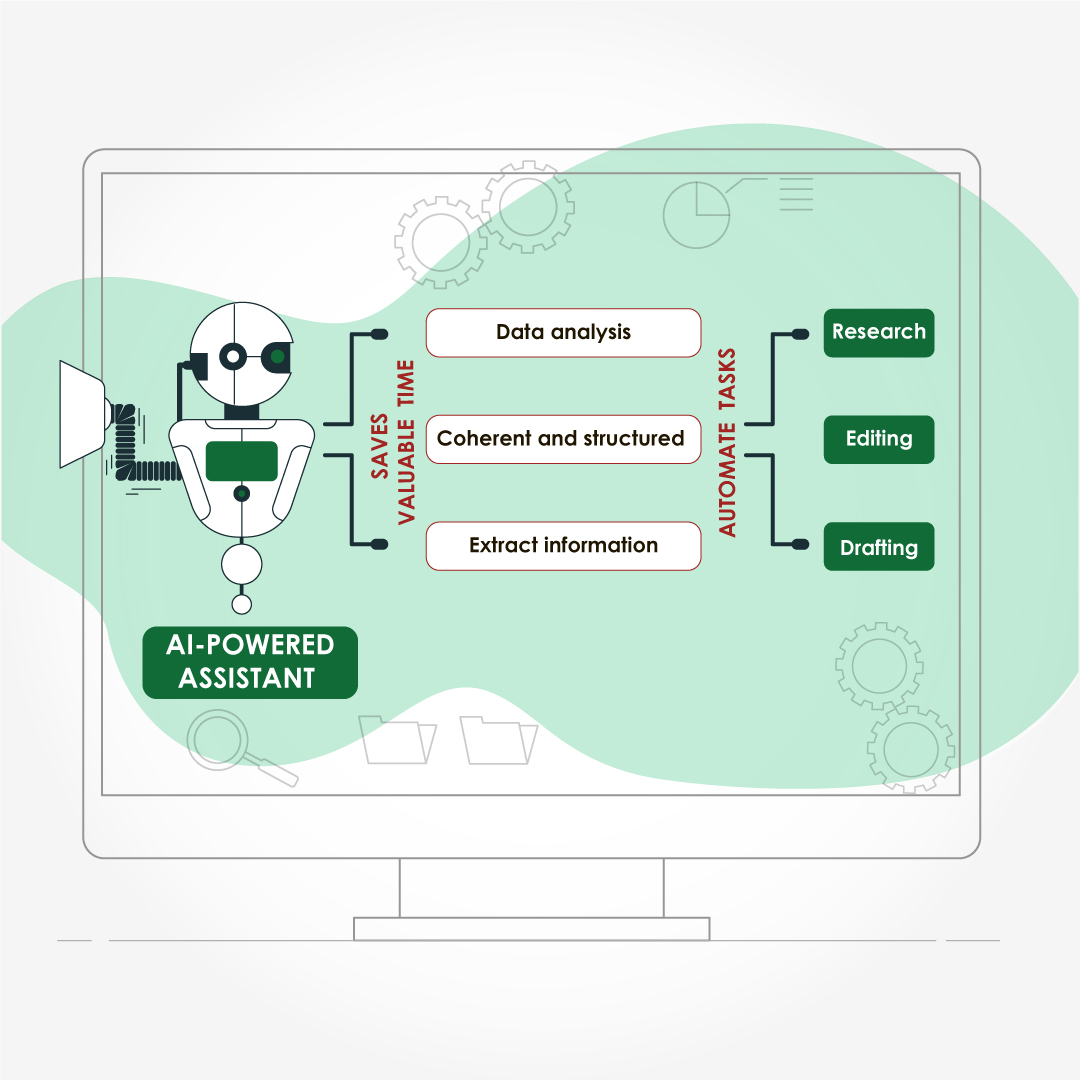
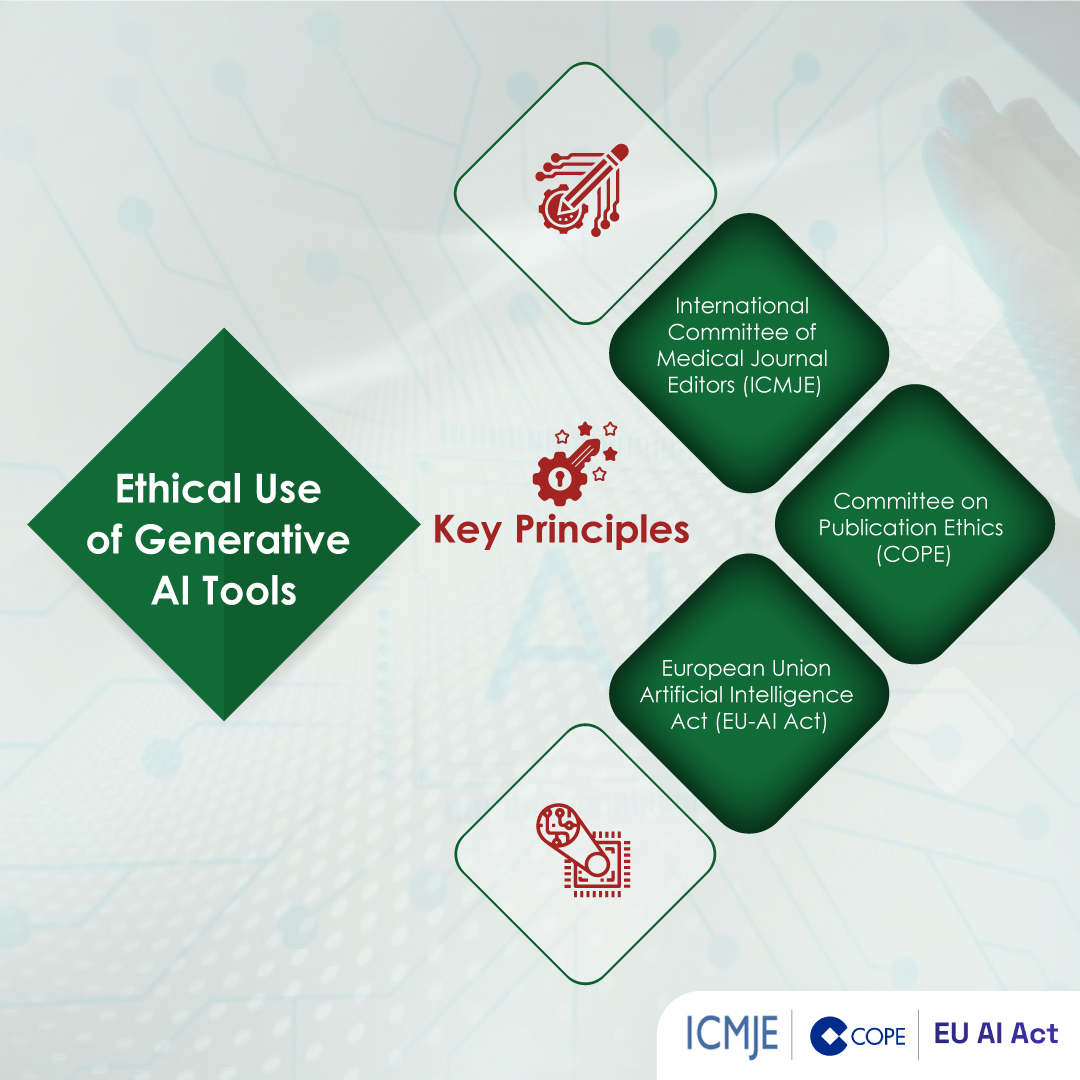





















 Module 1: Administrative and prescribing information
Module 1: Administrative and prescribing information

 Challenges and Considerations in CSR Preparation
Challenges and Considerations in CSR Preparation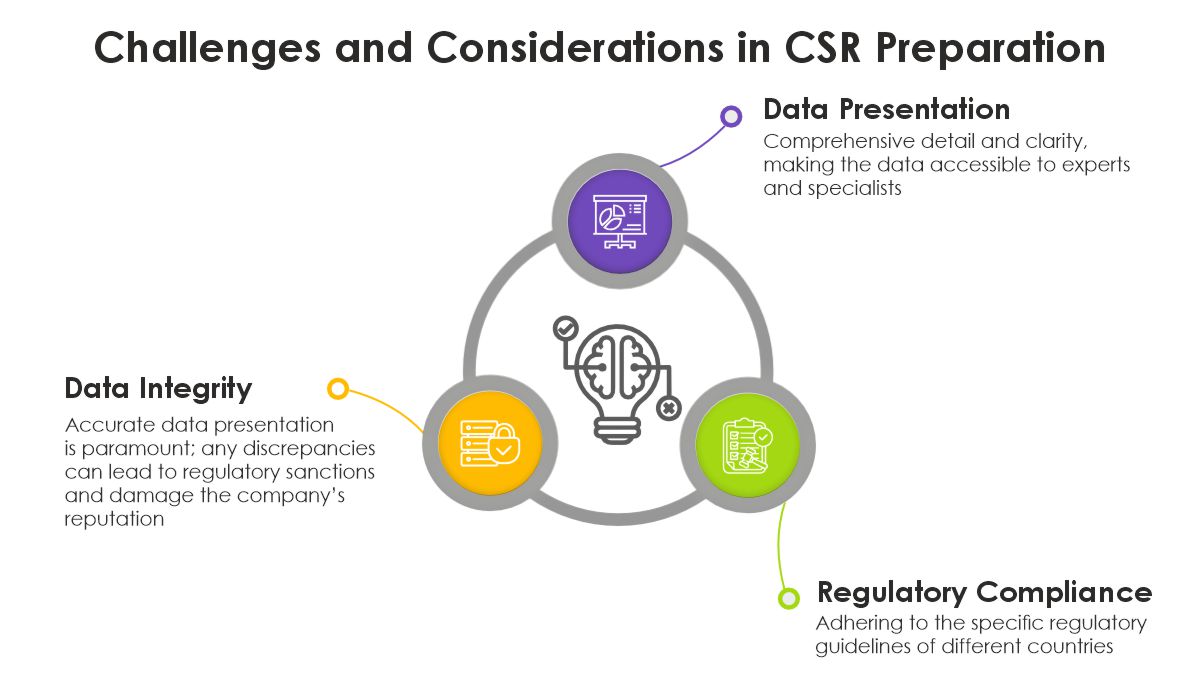 The Future of CSR
The Future of CSR