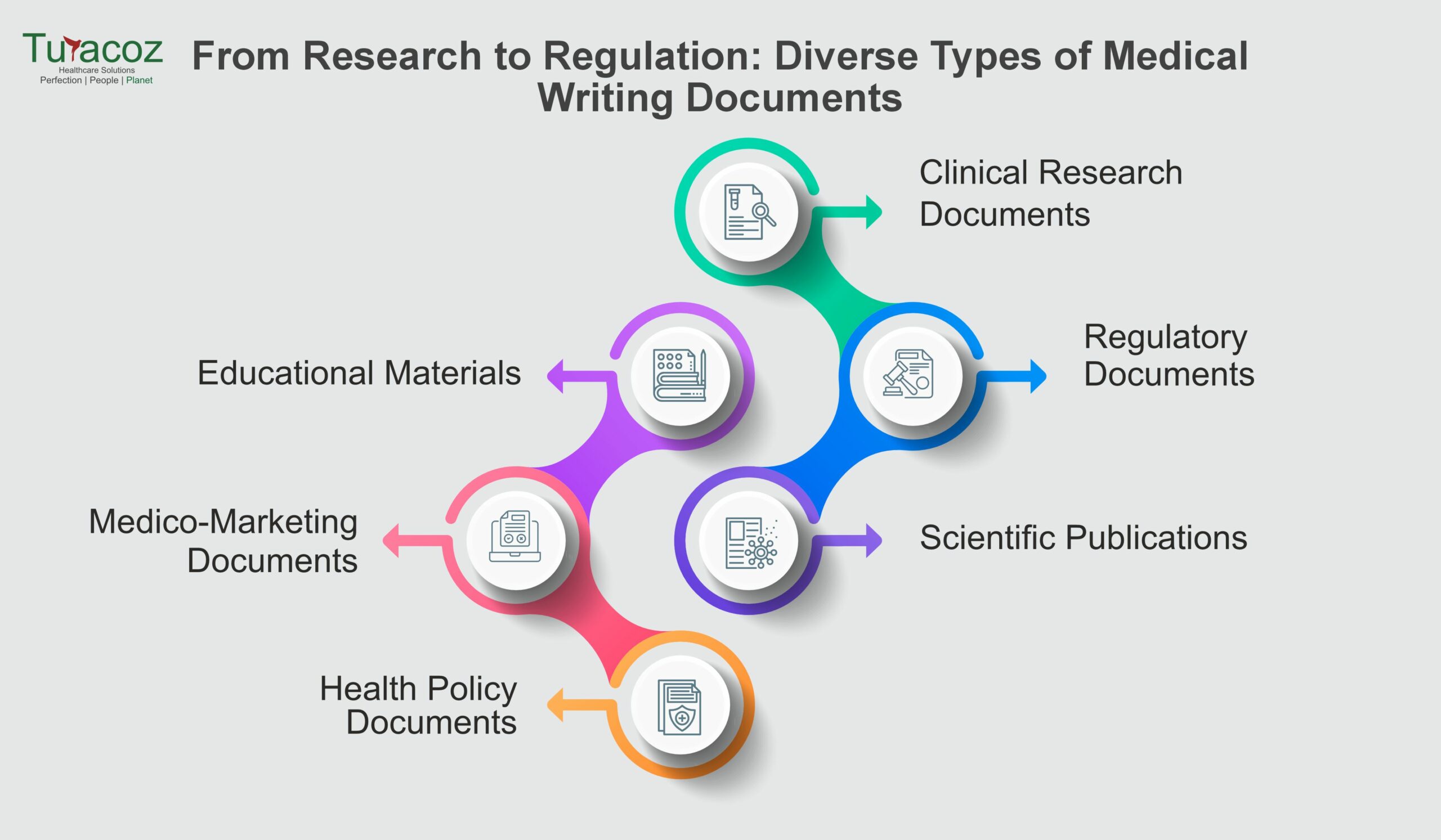
Medical writing plays a pivotal role in conveying medical information and research findings, addressing diverse needs within the healthcare and pharmaceutical sectors. This field encompasses a variety of documents, each serving specific purposes, from regulatory submissions and clinical research documentation to educational material and scientific publications. Here’s a closer look at our medical writing services and what kind of medical writing documents we provide:
Clinical Research Documents
Clinical Trial Protocols: These documents outline the objective, design, methodology, statistical considerations, and organization of a clinical trial, providing a roadmap for conducting the study.
Informed Consent Forms: Essential for clinical trials, these forms provide participants with information about the study, including its purpose, procedures, risks, benefits, and their rights as participants.
Clinical Study Reports (CSRs): Prepared after completing a clinical trial, CSRs present the methodology, results, and conclusions of the trial, comprehensively serving as a detailed record of the trial and its outcomes.
Patient Information Leaflets (PILs) and Summary of Product Characteristics (SmPCs): These are regulatory documents required for the marketing approval of a drug. PILs inform patients about how to use a medication, while SmPCs provide detailed information for healthcare professionals on how to prescribe it.
Regulatory Documents
Investigational New Drug (IND) Applications: These documents are submitted to regulatory authorities to obtain permission to initiate clinical trials with a new drug.
New Drug Applications (NDA)/Marketing Authorization Applications (MAA): Submitted to regulatory authorities to seek approval for marketing a new pharmaceutical for sale and use.
Periodic Safety Update Reports (PSURs): These reports offer updates on the safety profile of a pharmaceutical product, including any new risks identified.
Scientific Publications
Research Articles: These are detailed studies reporting original research, published in scientific journals.
Review Articles: Articles that summarize current research on a particular topic.
Case Reports: Detailed reports on the symptoms, diagnosis, treatment, and follow-up of an individual patient.
Abstracts and Posters for Scientific Conferences: Concise summaries of research findings presented at scientific meetings.
Educational Materials
Continuing Medical Education (CME) Materials: Designed to educate healthcare professionals about the latest developments in their field.
Patient Education Materials: Written in accessible language, these materials inform patients about their health conditions, treatment options, and healthy lifestyle choices.
Medico-Marketing Documents
Product Monographs: Detailed information about a drug, including its properties, indications, contraindications, side effects, etc.
Sales Training Manuals: Materials created to train sales representatives about the medical and scientific aspects of the products they will be selling.
Advertising Copy: Writing promotional materials targeting healthcare professionals or the public, adhering to regulatory standards.
Health Policy Documents
Health Technology Assessments (HTAs): Evaluations of the social, economic, organizational, and ethical issues of a health intervention or health technology.
Policy Briefs: Documents providing evidence-based recommendations on health policy issues to policymakers.
Turacoz Group is a leading medical writing agency and we provide different kinds of medical writing and academic writing services. If you are looking for a medical writer, you are at the right place. With us, you will get assistance with regulatory medical writing, research paper writing, academic writing, etc. We also provide medical and regulatory writing courses that are designed to equip you with essential skills and insights to excel in the dynamic healthcare industry. If you are intrigued by the world of medical writing and eager to learn how to create various document types, explore our “Comprehensive Certificate Course in Medical Writing.” Start your journey towards becoming a skilled medical writer today.
Reach out to us at [email protected] and elevate your expertise now!
Registration link: https://forms.office.com/pages/responsepage.aspx?id=TCiDEIoeEUeaAGbcYpnFeedjbeZ6M2hImsjsFwldXtdUNEpRVVlMRzZKUlpLTE1MUDA2NUdRQ0JVVC4u