Before the introduction of the Common Technical Document (CTD) in 2002, each major regulatory region, including the European Union (EU), the USA, and Japan, had its unique guidelines and formats for submitting regulatory dossiers to obtain marketing approval for new drugs or changes to existing licenses. For instance, Japan required the GAIYO, Europe needed Expert Reports and Tabulated Summaries, and the USA had specific FDA guidelines for New Drug Applications (NDA). These diverse requirements within the EU further complicated submissions, making the process highly repetitive and time-consuming across different countries and regions.
In 2000, to streamline this process, representatives from the European Medicines Agency (EMA), the USA FDA, and Japan’s Ministry of Health, Labour, and Welfare under the International Conference on Harmonisation (ICH) umbrella created unified guidelines for these dossiers. The CTD, first issued in 2002, aimed to reduce the time and resources needed to compile applications, simplify regulatory reviews, and improve communication with applicants through a standard format. It became the mandatory format for NDAs in the EU and Japan by July 2003 and was strongly recommended in the USA. Other countries including Canada and Switzerland have since adopted the CTD and are transitioning to an electronic format, the eCTD, mandatory in the EU for centralized procedures since 2010.
General Principle of CTD
- The ICH M4 guidelines for the CTD recommend clear and consistent formatting.
- Text and tables should be set with margins suitable for A4 and 8.5 × 11″ paper.
- Times New Roman, 12-point font is suggested for narrative text.
- Acronyms and abbreviations should be defined upon first use in each module, and literature references should be cited at the end of each module, following specific guidelines.
- Pages within the CTD should be sequentially numbered, except for literature references, and a unique header or footer briefly identifying the content of each page is required.
- The guidelines also allow for simplified section numbering to avoid excessive subheadings.
Overall organization of CTD
The CTD’s overall structure, outlined in the ICH M4 guidelines, includes a section detailing document location and pagination within the dossier, known as granularity. This guidance is beneficial for dossiers containing multiple indications or components of the investigational medicinal product (IMP). Additionally, question-and-answer documents accompany the M4 guidelines to address common issues. The CTD dossier comprises five main modules: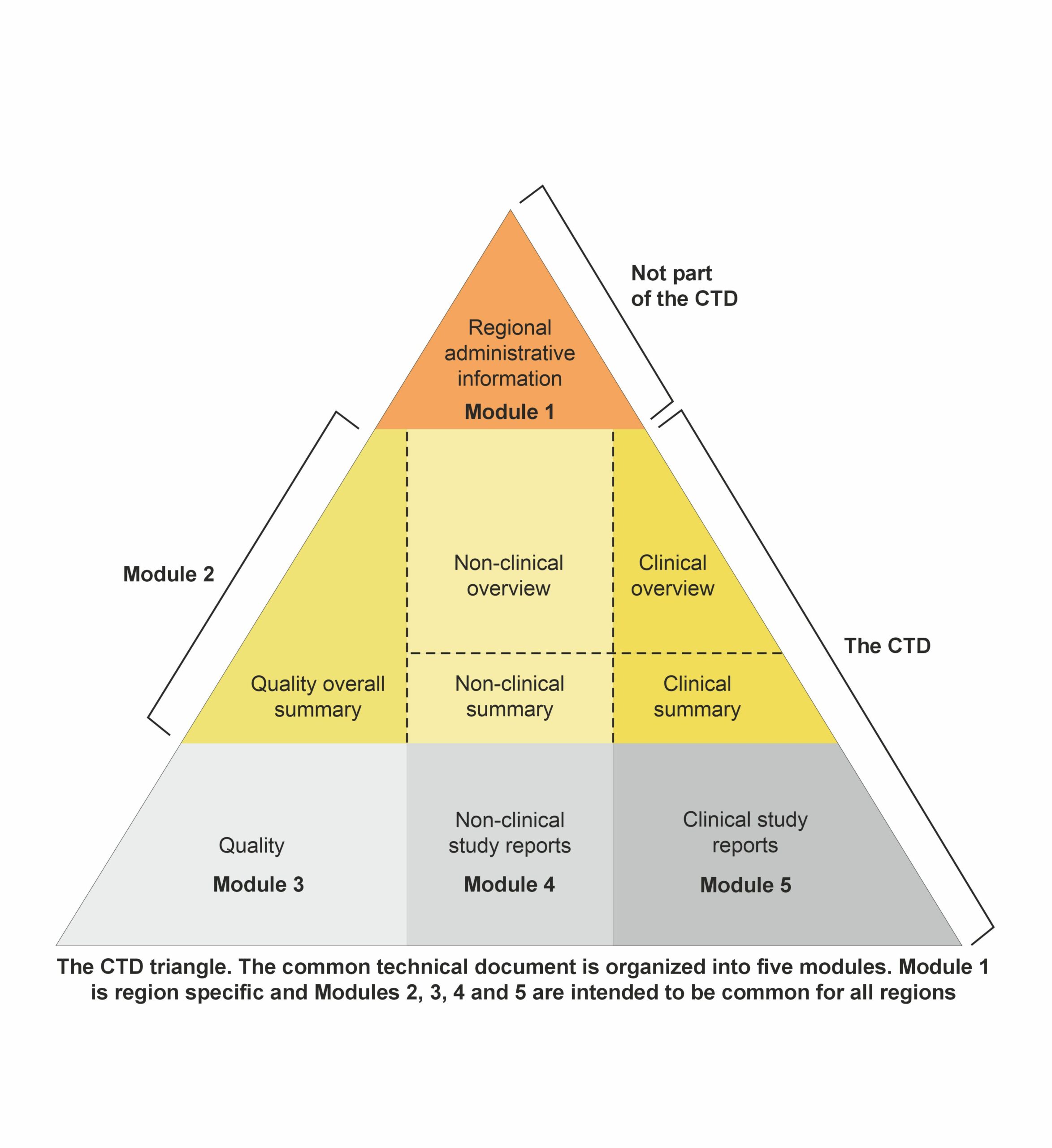
It is region-specific and includes all administrative documentation and prescribing information required by the local regulatory authority. This module comprises application forms, the proposed labeling, environmental risk assessments, and manufacturer details tailored to meet local legal and regulatory mandates. It sets the stage for the evaluation process by providing essential administrative and information.
Modules 2–5though, are standard to all regions, and these comprise the main body of the CTD.
Module 2: Overviews and Summaries of Modules 3–5
Module 2 consists of seven sections organized as follows:
- 2.1: Table of contents
- 2.2: Introduction:
Provides a brief overview of the IMP, including its pharmacological class, mode of action, and proposed clinical use. In general, the introduction should not exceed one page.
- 2.3: Quality overall summary:
- A summary of chemical and pharmaceutical data in the dossier, following the structure of Module 3.
- Aimed at discussing critical parameters and addressing development issues.
- Typically, not exceeding 40 pages, excluding tables and figures.
- 2.4 and 2.6: Non-clinical overview and written/tabulated summaries:
- Detailed in the ICH M4S guidelines, comprehensive summaries of pharmacology, pharmacokinetics, and toxicology are provided.
- The non-clinical overview is an integrated assessment, not exceeding 30 pages.
- The written summaries are around 100-150 pages, with tabulated summaries using provided templates.
- 2.5 and 2.7: Clinical overview and summary:
- Specified in the ICHM4E guidelines, offering critical assessment and factual summarization of clinical data.
- The clinical summary ranges from 50 to 400 pages, focusing on factual observations.
- The clinical overview, which is around 30 pages, provides critical analysis and discusses the development program, efficacy, safety, and risk/benefit conclusions.
Click Here:- The Power of Visualization: How Scientific Diagrams, Graphs, Videos, Infographics Boosts Publications and Citations
Module 3: Quality documentation
It contains the chemistry, manufacturing, and controls reports essential for the product’s registration dossier. The specific requirements for Module 3 are outlined in detail in the ICH M4Q guideline. This module comprises sections covering both the drug substance and the drug product. The main headings within Module 3 must remain unchanged.
- 3.1: Table of Contents
- 3.2: Body of Data
- 3.2.S: Drug Substance
- 3.2. P+: Drug Product
- 3.3: Literature References used within Module 3
Module 4: Non-clinical reports on pharmacology and toxicology
Module 4 comprises the non-clinical reports incorporated into the dossier. The organization and content of Module 4 are delineated in the ICH M4S guidelines. The primary headings within this section should remain unchanged.
- 4.1 Table of contents of Module 4
- 4.2 Study reports
- 4.2.1 Pharmacology
- 4.2.2 Pharmacokinetics
- 4.2.3 Toxicology
- 4.3 Literature references used in Module 4.
Module 5: Clinical study reports
Module 5 showcases the clinical reports incorporated into the dossier. The organization and content of Module 5 are outlined in the ICH M4E guidelines, which offer precise instructions for the placement of clinical study reports and related information to streamline preparation and review processes and guarantee completeness. A report’s positioning is dictated by the study’s primary objective, with each report appearing in only one section. In cases of multiple objectives, the study should be cross-referenced in the appropriate sections. The principal headings within this section, which should remain unchanged, include:
- 5.1 Table of contents of Module 5
- 5.2 Tabular listing of all clinical studies
- 5.3 Clinical Study Reports
- 5.3.1 Reports of biopharmaceutic studies
- 5.3.2 Reports of studies pertinent to pharmacokinetics using human biomaterials
- 5.3.3 Reports of human pharmacokinetic (PK) studies
- 5.3.4 Reports of human pharmacodynamic (PD) studies
- 5.3.5 Reports of efficacy and safety studies
- 5.3.6 Reports of post-marketing experience
- 5.3.7 Case report forms and individual patient listings
- 5.4 Literature references.
This module is pivotal in demonstrating the drug’s therapeutic benefits outweigh its risks, supporting its clinical utility.
Conclusion
The CTD format is a cornerstone of modern drug regulatory submissions, facilitating a smoother, more efficient review process that benefits regulatory authorities, pharmaceutical companies, and ultimately, patients. By standardizing the format and content of drug dossiers, the CTD helps ensure that high standards are maintained, and that new, effective, and safe medicines are made available to the public promptly. Understanding and mastering the structure of the CTD is essential for any pharmaceutical professional involved in the global submission of drugs for approval.
For those interested in taking their first steps into medical writing or enhancing their expertise to meet the challenges of omnichannel communication in healthcare, we invite you to explore the opportunities available through our training programs. Contact us at [email protected] to learn how you can join the ranks of medical writers making a significant impact in healthcare communication. Together, let’s shape a future where accurate, accessible, and actionable health information reaches every corner of the globe, empowering individuals and transforming healthcare outcomes.































