In clinical research, documenting results and findings is as important as conducting the study. The Clinical Study Report (CSR) is crucial in this process. It is essential to evaluate the safety and effectiveness of new drugs and therapies, whether you are a healthcare professional, part of the pharmaceutical industry, or a regulatory agency. This blog will explore what a CSR is, its components, its importance, and its role in the regulatory approval process.
What is a Clinical Study Report?
A CSR is a detailed document presenting a clinical trial’s methodology, results, and conclusions. It is designed to provide a comprehensive account of the trial, ensuring that the data generated is transparent, replicable, and accessible. Regulatory authorities primarily use CSR to make informed decisions regarding approving a drug or therapy for market release.
Structure of CSR
The format and content of a CSR are generally guided by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), precisely ICH E3 guidelines. These guidelines help standardize the reports across different trials and pharmaceutical companies, facilitating more accessible reviews by regulatory bodies. A typical CSR includes the following key sections:
- Title Page and Synopsis: This section provides basic information about the study, including its title, phase, and summary of the trial and its outcomes.
- Table of Contents: Lists the major and minor sections of the document for easy navigation.
- List of Abbreviations and Definitions: Clarifies terms and abbreviations used throughout the report.
- Trial Protocol and Amendments: This section includes the original study protocol and any amendments made during the study. It outlines the study objectives, design, population, treatments, procedures, and statistical considerations.
- Trial Participants: Detailed information about trial participants, including inclusion and exclusion criteria, demographic data, and the number of participants who completed or withdrew from the trial.
- Efficacy and Safety Results: This is often the most scrutinized section of the report. It details the trial’s outcomes, including primary and secondary endpoints, statistical analyses, and efficacy and safety data.
- Discussion and Conclusion: The authors interpret the results and discuss the implications for future research and clinical practice. The conclusions must be supported by the data presented in the report.
- Appendices: These may include raw data tables, sample case report forms, and details of the statistical analysis methods.
Types of CSR
CSR can be categorized based on the trial stage, the report’s purpose, and its audience.
- Full Reports: These are the most comprehensive type of CSR and include complete details about the trial’s design, methodology, statistical analysis, results, and conclusions. Complete reports are necessary for regulatory submission and are scrutinized by regulatory authorities as part of the drug approval process.
- These reports are shorter and include summarized data. They are often used for internal Abbreviated Reports: documentation and preliminary discussions with regulatory authorities to give an overview of the trial outcomes without going into the depth typically required for final approval.
- Interim Reports: During ongoing trials, interim reports provide a snapshot of the trial’s progress. They are crucial for long-term studies and are often used to make decisions about the continuation or modification of the trial. Interim reports can be critical in adaptive clinical trials, where the data from these reports can influence future trial phases.
- Safety Reports: Focused specifically on the safety outcomes of a clinical trial, safety reports detail adverse events, serious adverse events, and other safety data collected during the study. They are crucial for ongoing safety evaluations by regulatory bodies and ethics committees.
- Integrated Reports: These reports compile data from multiple studies of a single drug or therapeutic approach. Integrated reports are helpful for regulatory submissions when a comprehensive view of a drug’s efficacy and safety is required across various populations, dosages, or comparators.
- Pharmacokinetic/Pharmacodynamic (PK/PD) Reports: Specialized reports that focus on pharmacokinetics (how the drug moves through the body) and pharmacodynamics (the drug’s effects on the body) are crucial for understanding the dosing and mechanism of action of a drug.
- Statistical Reports: These provide detailed statistical analyses and interpretations separate from the main body of clinical findings. They are essential for specialists assessing the statistical validity of the trial results.
- Patient Reports: Sometimes required by regulatory authorities, these reports provide a summary of the clinical trial and its results in a format that is accessible to patients and the general public. This type is becoming increasingly important as patient advocacy groups call for greater transparency in clinical research.
Importance of CSRs
CSRs play a critical role in the pharmaceutical and medical fields for several reasons:
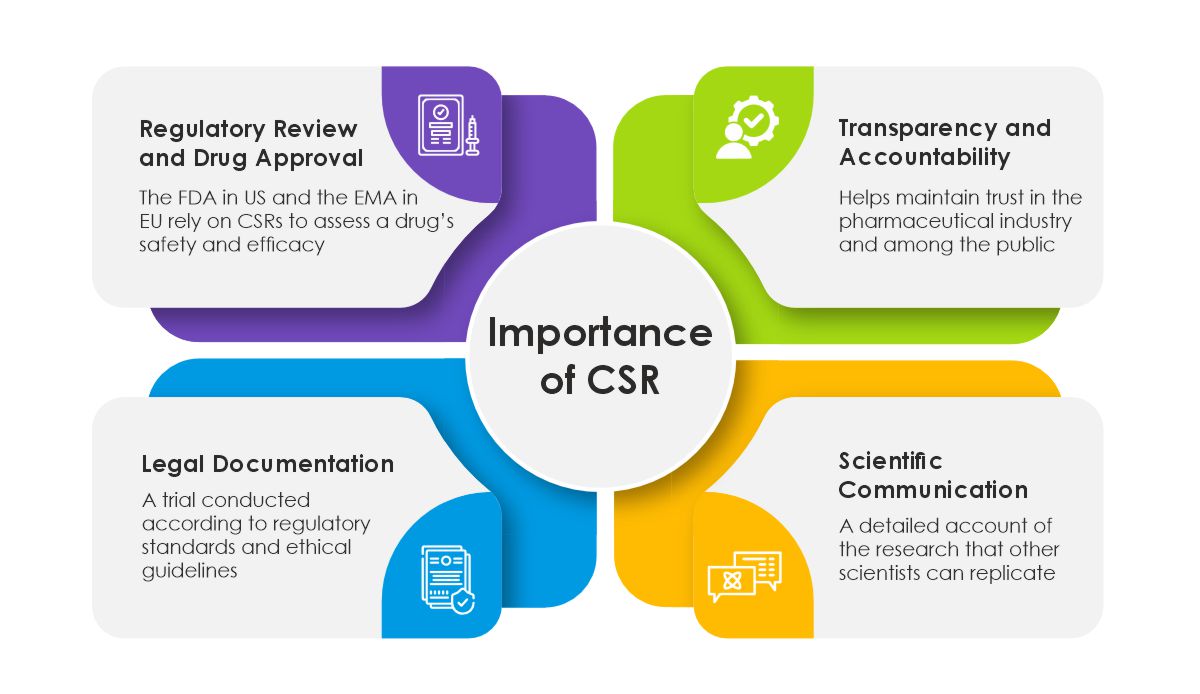
Regulatory Review and Drug Approval: CSRs are fundamental in drug approval. Regulatory bodies like the FDA in the United States and the EMA in Europe rely heavily on the information provided in CSRs to assess a drug’s safety and efficacy.
Transparency and Accountability: By detailing every aspect of the clinical trial, CSRs ensure transparency in clinical research. This helps maintain trust in the pharmaceutical industry and among the general public.
Scientific Communication: CSRs are often used as a basis for publications in scientific journals. They provide a detailed account of the research that other scientists can replicate or build upon.
Legal Documentation: In cases of legal scrutiny, CSRs serve as an essential document to verify that a trial was conducted according to regulatory standards and ethical guidelines. Challenges and Considerations in CSR Preparation
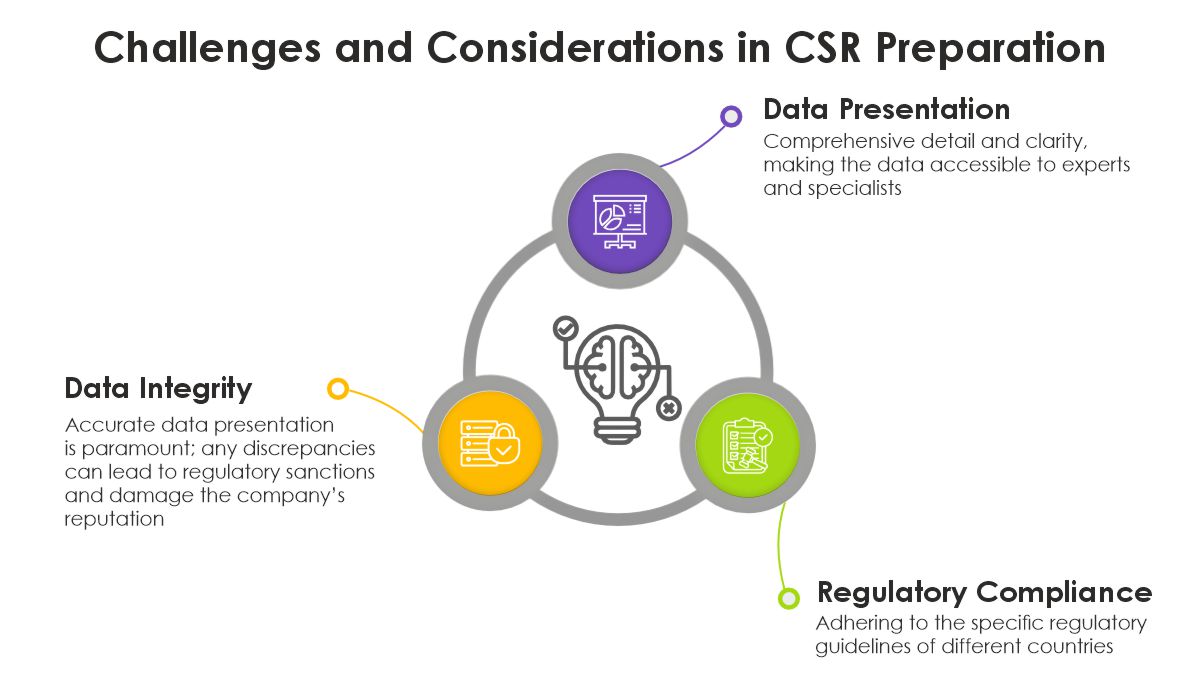
Challenges and Considerations in CSR Preparation
- Data Integrity: Ensuring that the data presented in the CSR accurately reflects the findings from the trial is paramount. Any discrepancies can lead to regulatory sanctions and damage the company’s reputation.
- Compliance with Regulations: Adhering to the specific regulatory guidelines of different countries can be complex, especially for multinational trials.
- Clarity and Detail: The CSR must strike a balance between comprehensive detail and clarity, making the data accessible not just to experts but also to those who may not be specialists in the field.
With advancements in digital technology, the future of CSRs may include more interactive and dynamic formats. Regulatory agencies are also moving towards greater transparency, with some requiring the publication of full CSRs online to facilitate public access and scrutiny.
In conclusion, Clinical Study Reports are not just documents but the backbone of clinical research transparency, regulatory review, and scientific communication. They ensure that the medical and patient communities can trust the results and safety of new therapies. As clinical research continues to evolve, so will the standards and practices surrounding CSRs, enhancing their role in advancing healthcare and treatment options globally.
For those interested in taking their first steps into medical writing or enhancing their expertise to meet the challenges of omnichannel communication in healthcare, we invite you to explore the opportunities available through our training programs. Contact us at trainings@turacoz.com to learn more about how you can join the ranks of medical writers making a significant impact in healthcare communication. Together, let’s shape a future where accurate, accessible, and actionable health information reaches every corner of the globe, empowering individuals and transforming healthcare outcomes.


 Challenges and Considerations in CSR Preparation
Challenges and Considerations in CSR Preparation



























