Types of Response Documents That
Our Team Have Created Till Now




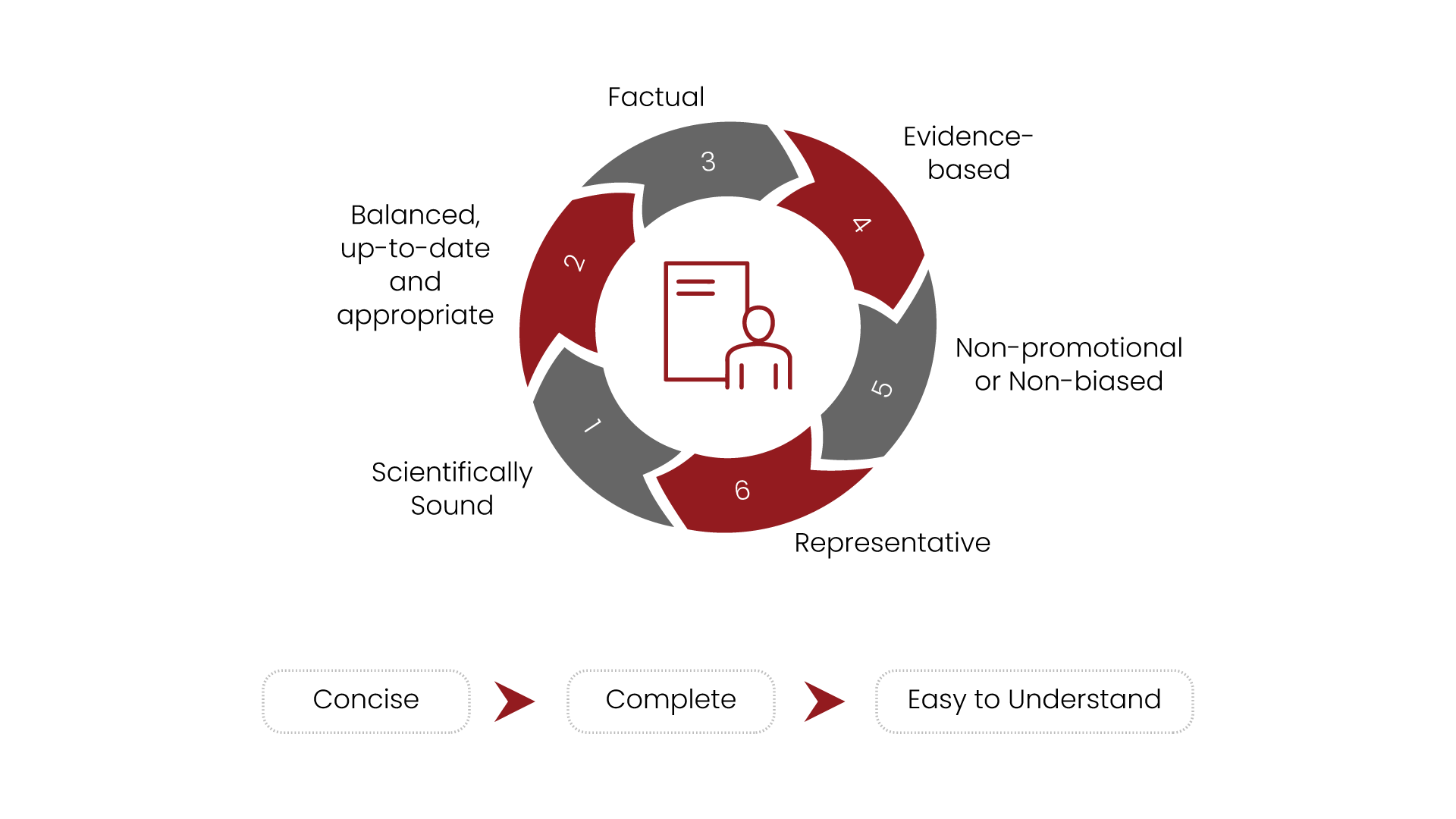
Common Features of Response Documents
Claim Substantiation and
Science Consulting
Claims for a regulated product are the promise to the user for its performance, safety, and use. As with all communication about a product, the claim statements can be found in many different documents and communications.
Right from the product label to product brochure, or an advertisement or company’s website, or science dossiers, claims can be found everywhere.
At Turacoz, we have a dedicated team working on this deliverable type.