Scientific research dissemination has undergone a significant transformation in recent years, largely owing to the rise of preprint repositories. Preprints, which are versions of scholarly papers that precede formal peer reviews and publications in academic journals, have become an integral part of the scientific communication ecosystem. This blog explores the rise of preprint repositories, their role in accelerating research dissemination, and their impact on traditional journal publishing.
The Rise of Preprint Repositories
They have emerged as vital platforms on which researchers can share their findings with the global scientific community. Repositories, such as arXiv, bioRxiv, and medRxiv, have become popular across various disciplines. The concept of preprints is not new; it dates to the early 1990s with the launch of arXiv, a repository for physics research. However, the proliferation of preprint servers across different fields is a relatively recent phenomenon driven by the need for quicker dissemination of scientific knowledge. The coronavirus disease 2019 (COVID-19) pandemic has accelerated the adoption of preprints. With the urgency to share critical research findings related to the virus, treatments, and vaccines, preprint repositories have played a crucial role in providing immediate access to scientific data. This shift highlights the importance of preprints in responding to global health emergencies and underscores their potential to expedite the research process.
Accelerating Research Dissemination
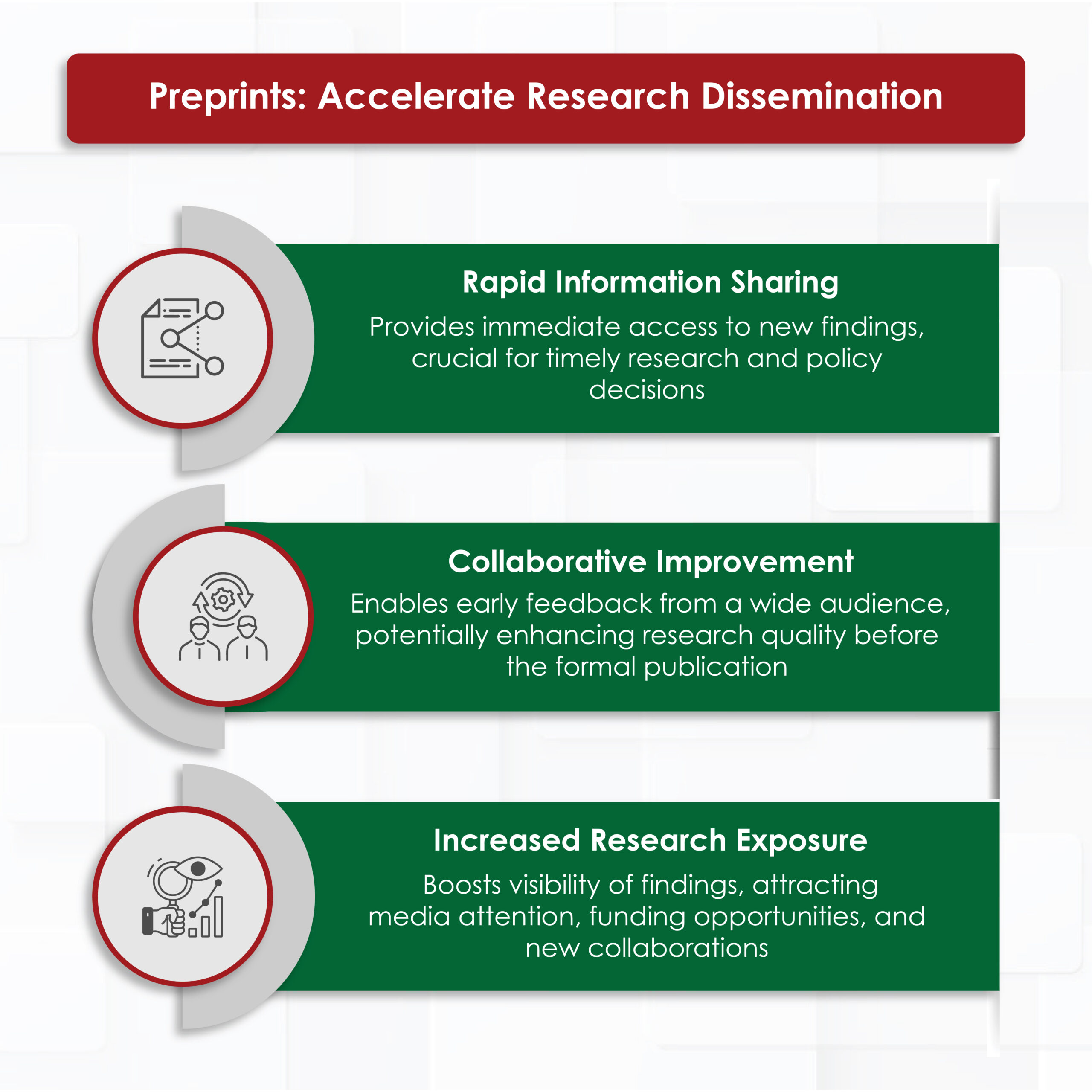
Preprints have revolutionized the speed at which research findings are shared. Traditionally, peer review and publication in academic journals can take months if not years. This delay can hinder the timely dissemination of important scientific discoveries. Preprints address this issue by allowing researchers to share their work with the community almost immediately after completing their manuscript. Rapid dissemination has several benefits:
- Immediate Access to Research: Researchers, clinicians, and policymakers can access the latest findings without waiting for a lengthy peer review process. This immediate access is particularly crucial in fields in which timely information can influence ongoing research and public health decisions.
- Increased Collaboration: By making research publicly available at an early stage, preprints foster collaboration and feedback from a broader audience. Researchers should receive constructive comments and suggestions to improve the quality of their work before formal publication.
- Enhanced Visibility: Preprints can increase the visibility of research findings. Studies available as preprints can attract the attention of the media, funding agencies, and other researchers, potentially leading to new opportunities for collaboration and funding.
Impact on Traditional Journal Publishing
The rise of preprints has significant implications for traditional journal publishing. While preprints offer several advantages, they also challenge the established norms of scientific communication.
- Peer Review Process: One of the primary roles of academic journals is to provide rigorous peer review to ensure the quality and reliability of published research. Preprints, by definition, are not peer-reviewed, which has raised concerns about the potential spread of misinformation and the and credibility of unreviewed findings. However, many preprint servers have implemented basic screening processes to mitigate these concerns.
- Citation and Credibility: The acceptance and citation of preprints in academic circles have been the subject of debate. Some researchers and institutions hesitate to cite preprints, preferring peerreviewed articles for their credibility. However, the scientific community is gradually recognizing the value of preprints, and many funding agencies and institutions now consider preprints in grant applications and tenure evaluations.
- Economic Model: The conventional journal publishing model, which is based on subscription fees and article processing charges, faces challenges from the open-access nature of preprints. Preprints offer an alternative that can reduce the financial burden on researchers and institutions. This shift has prompted journals to explore new business models and to consider the integration of preprints into their publication pipelines.
Preprints in Medical Research
Preprints are particularly influential in medical research. The importance of sharing findings quickly in the medical field, where discoveries can directly impact patient care and public health policies, cannot be overstated. Preprint repositories like medRxiv have gained prominence, providing a platform for medical researchers to disseminate their work before formal peer review.
However, the use of preprints in medical research also requires careful consideration. Since unreviewed medical research can influence clinical practice and public health decisions, a balanced approach is necessary. Researchers and readers must exercise caution, critically evaluating the credibility and reliability of preprint findings.
Future Directions
The incorporation of preprints into the scientific communication landscape will likely continue to evolve. Several trends and developments can be anticipated:
- Enhanced Review Mechanisms: Preprint servers may develop more potent review mechanisms, including post-publication peer review and community-based feedback systems. These enhancements can improve the quality and reliability of preprints while retaining the speed of dissemination.
- Integration with Journals: Some academic journals are exploring partnerships with preprint servers, offering streamlined submission processes that allow researchers to submit preprints directly to journals for peer review. This integration can bridge the gap between preprints and traditional publishing.
- Policy and Guidelines: Institutions, funding agencies, and publishers are likely to develop clearer policies and guidelines regarding the use of preprints. Standardized practices can help address concerns about the credibility and citation of preprints.
- Education and Awareness: As preprints become more prevalent, educating researchers, clinicians, and the public about their proper use and interpretation will be essential. Increased awareness can help mitigate the risks associated with unreviewed research while maximizing the benefits of rapid dissemination.
Preprints have transformed the way scientific research is shared and accessed, offering a faster, more collaborative approach to dissemination. Their rise has had a profound impact on traditional journal publishing, challenging established norms and prompting innovation in the peer review process. As the scientific community continues to embrace preprints, their role in accelerating research dissemination and enhancing public engagement with science is likely to expand, shaping the future of scientific communication.
At Turacoz, we specialize in assisting researchers with the preparation of preprints. Our team of experienced medical writers and reviewers ensures that your preprints are clear, accurate, and impactful, maximizing their potential to reach and engage a broad audience. Visit www.turacoz.com or contact at [email protected] to learn more about how we can support your research communication needs.











